Expertise
What is Quantum Chemistry Molecular Modeling?
-
Verification and prediction of chemical phenomena in the molecular van der Waals force assembly system using the density functional theory concerning surface molecular electron density.
-
The energy structure of the van der Waals force assembly system can be visualized by electromagnetic wave energy absorption spectra (UV-Vis, NIR, IR, FIR, NMR, Raman spectra).
- Let’s consider molecular modeling for what happen between hydrogen (H2) and hydroxyl radical (HO.) in water. H2, HO. and H2O may aggregate one another by van der Waals & Coulomb interactions (hereinafter abbreviated as vdW), which collectively refers to hydrogen bonds and ion dipole interactions. It is considered that such a molecular aggregation constitutes a possible molecular assembly system in water.
- Quantum chemistry molecular modeling, that is, molecular modeling based on density functional theory (DFT/MM), can be performed using a personal computer (PC) that has reached the level of a former supercomputer. In other words, by using Spartan 18 developed by Wavefunction Inc / Q-chem Inc, anyone can perform mathematical analysis of electron orbitals involved in the bonds between vdW associated molecules.
- Obtaining the three-dimensional structure of the aggregate [H2&HO.&H2O] is to verify and predict the transition state of the reaction and the reaction intermediate. By calculating the structure (single point, SPE) obtained by the molecular mechanics method (MMFF) of a molecular assembly, the reaction transition structure of HO.&H2&H2O (SPE) will become apparent (Case 1-1). The equilibrium geometry HO.&H2&H2O (EQG) obtained by DFT/MM corresponds to a possible reaction intermediate structure (Case 1-2).
- In DFT/MM, the energy structure of the molecular orbitals of the molecular aggregate is visualized by the electromagnetic wave energy absorption spectrum, i.e. UV/Vis spectrum. The UV/Vis spectra can be called theory spectra. By comparing the measured UV/Vis spectrum with the theory spectrum, it is possible to verify and predict the equilibrium aggregate structure (Case 2).
- Electromagnetic wave energy absorption spectrum refers to not only UV / Vis, but also Near IR, IR, FIR, MW, Radio wave, NMR spectra. In the theory UV/ Vis obtained by DFT/MM, it is possible to know the attribution of the transition between the highest occupied orbital group (HOMO) and the lowest empty orbital group (LUMO) with respect to the absorption maximum and the transition probability indicating the intensity thereof.
- It is worth noting that aggregates of inorganic and organic molecules with radicals and unpaired electrons can also be analyzed by DFT / MM (Case 3).
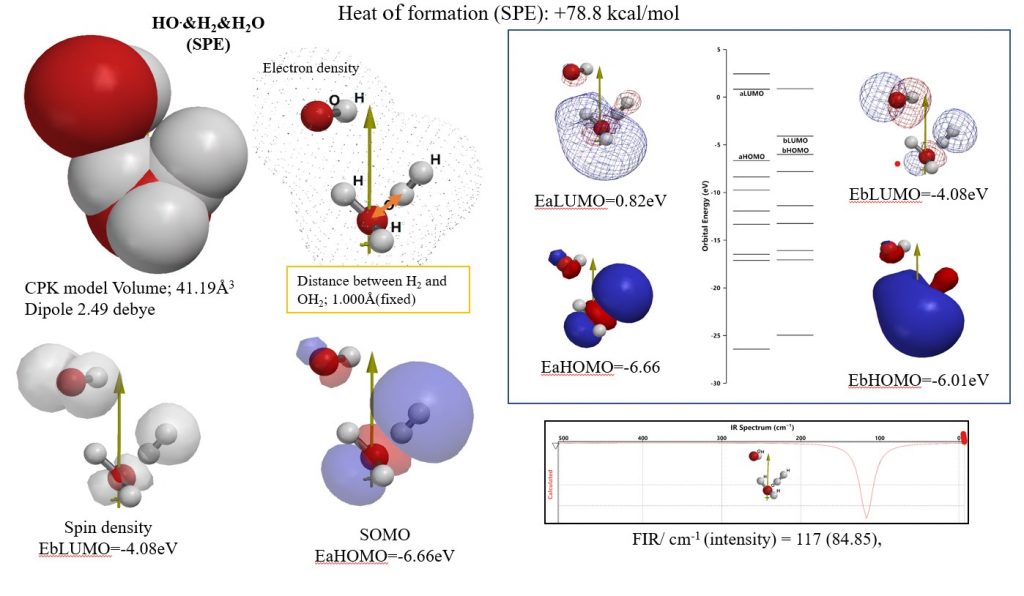
Example 1-1 of quantum chemical molecule modeling:
The aggregate structure HO. & H2 & H2O (SPE) obtained by single point energy calculation can be regarded as the reaction transition state.
In order to answer the question of whether hydrogen molecules react with hydroxyl radicals in the cellular environment in the body, the structure of van der Waals aggregates of HO radical, H2 and H2O is determined by molecular mechanics (see the figure below). The single point energy for the structure was calculated. The heat of formation (SPE) obtained at that time is endothermic and is required to be +77.8 kcal / mol. We verify that the energy structure of the molecular orbital is easily reduced and easily oxidized (EbLUMO, and EbHOMO) . From the theory FIR spectrum, it was verified that the molecule aggregation proceeds exothermically.
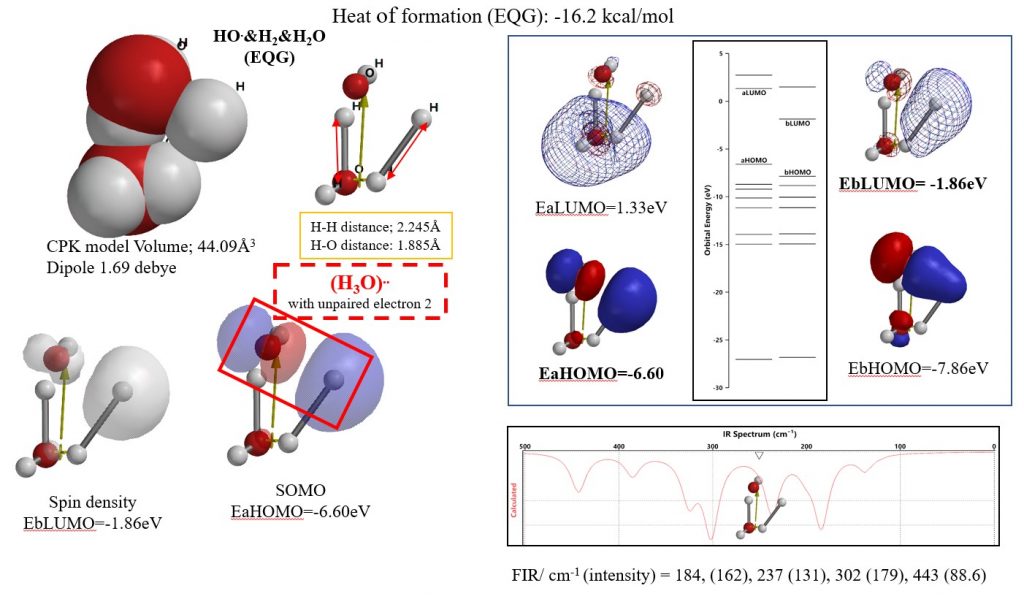
Example 1-2 of quantum chemistry molecule modeling:
Equilibrium geometry [HO. & H2 & H2O (EQG)] can be regarded as a reaction intermediate in which the cleaved hydrogen molecule exhibits reducing power to HO.
The Equilibrium geometry structure for the SPE structure of the van der Waals assembly of HO radical, H2 and H2O was determined. The Heat of formation obtained at that time was exothermic and was -16.2 kcal / mol. In the EQG structure, hydrogen molecules are in a cleaved state, and the theoretical FIR spectrum shows that the reaction proceeds exothermicly by absorption of electromagnetic energy. The formation of an associated intermediate [(H3O) ..] between a cleaved hydrogen molecule and a hydroxyl radical can be predicted.
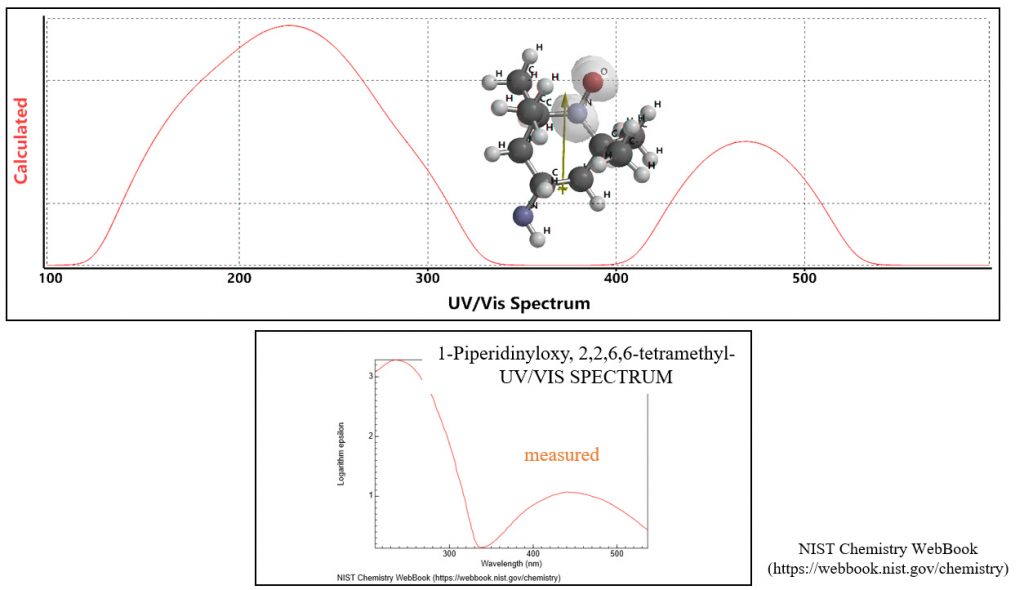
Example of quantum chemistry molecule modeling 2:
Theory UV-Vis spectrum of radical molecules agrees with the measured UV-Vis spectrum
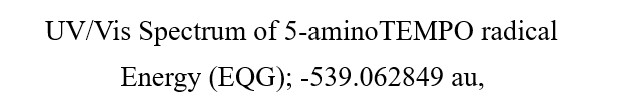

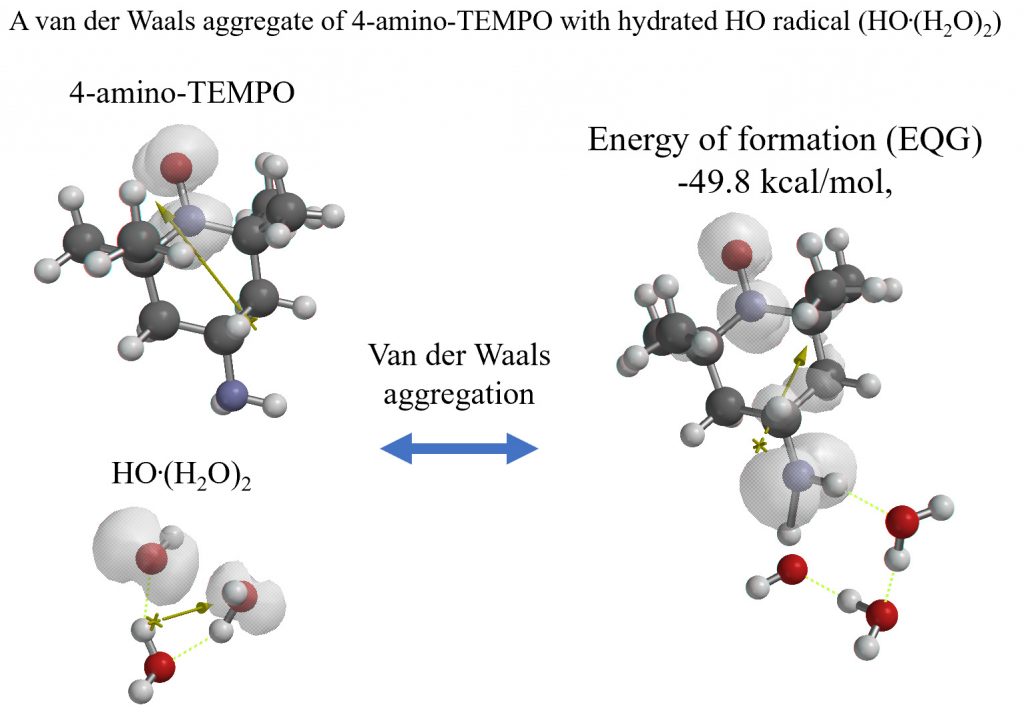
Example of quantum chemistry molecule modeling 3:
Verification that hydroxyl radical reacts with radical trapping agent 4-amino-TEMPO to stabilize radical sites